Crystal Field Theory
Crystal Field Theory: Overview
This Topic covers sub-topics such as Spectrochemical Series, Jahn Teller Effect, Strong Field Ligands, Crystal Field Splitting in Octahedral Complexes, Colour in Coordination Compounds and, Crystal Field Splitting in Tetrahedral Complexes
Important Questions on Crystal Field Theory
: [Ti(H2O)6]4+ is coloured while [Sc(H2O)6]3+ is colourless.
: d - d transition is not possible in [Sc(H2O)6]3+
The configuration of central atom is for octahedral complex. The correct statement about this compound.
Which of the following is the strongest ligand?
What are the exceptions to Crystal Field Theory (CFT) ?
Crystal Field Theory takes only d-orbitals of a central atom into account. The s and p orbits are not considered for the study.
Crystal Field Theory fails to explain the behaviour of certain metals which cause large splitting while others show small splitting.
Crystal Field Theory does not take into account the covalent character of bonding between the ligand and the central atom.
Crystal Field Theory does not take into account the _____ (ionic/covalent) character of bonding between the ligand and the central atom.
are _____ (strong/weak) field ligands.
Which of the following is strong field ligand?
are _____ (strong/weak) field ligands.
Which of the following is weak field ligand?
The crystal field theory could not explain why anionic ligands are found at the low end of the _____ series.
Which of the following is incorrect?
The crystal field theory does not consider the _____ character in metal-ligand bonding.
Write any two limitation of crystal field theory.
A complex of metal has the following electronic distribution in orbitals,
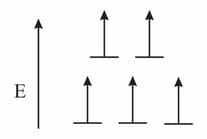
The neutral M has a ground state electronic configuration of Which of the following complexes is consistent with the electronic distribution of (as described above)?
Which of the following compounds is not coloured?
An octahedral complex with magnetic moment may have:
(a) configuration with weak field ligand.
(b) configuration with strong field ligand.
(c) configuration with strong field ligand.
(d) configuration with weak field ligand.
Crystal field splitting energies for octahedral and tetrahedral geometries caused by the same ligands are related through the expression
